Olive Diagnostics, an Israeli medical device startup, has completed a successful clinical trial for its KG, the world’s first artificial intelligence (AI)-based optical device capable of early detection of diseases at home or in the clinic.
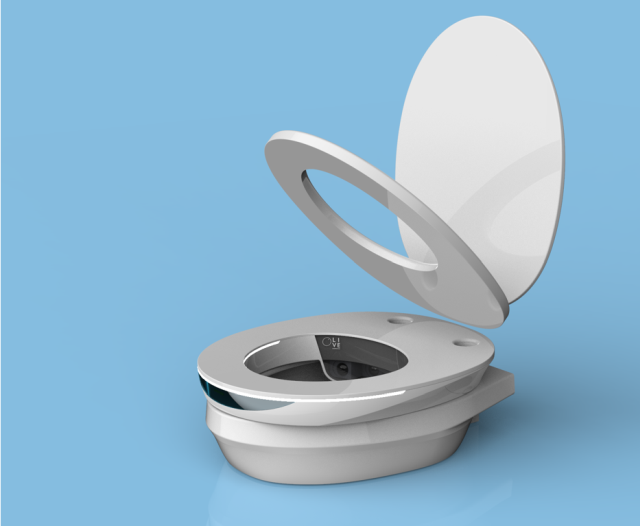
Olive Diagnostics’ equipment, which attaches to any toilet, accurately detects biomarkers for a variety of common medical ailments and diseases, including some types of prostate, ovarian, and kidney malignancies; heart failure; dehydration; kidney stones; and inflammation of the urine bladder.
KG will send medical information to family members and caregivers in order to detect the risk of dehydration and provide insight into people’s bowel movements. The KG device is an essential tool for the elderly, where early detection can significantly improve both qualities of life and care.
Will you offer us a hand? Every gift, regardless of size, fuels our future.
Your critical contribution enables us to maintain our independence from shareholders or wealthy owners, allowing us to keep up reporting without bias. It means we can continue to make Jewish Business News available to everyone.
You can support us for as little as $1 via PayPal at office@jewishbusinessnews.com.
Thank you.
Olive Diagnostics’ KG is a low-cost Internet of Medical Things (IoMT) that analyzes the molecular composition of urine in real-time and delivers secure and tailored diagnostic data.
The device generates data on parameters such as red blood cells, protein, ketone, and creatinine, as well as other characteristics of the urine such as volume, pressure, color, and frequency.
The Olive Diagnostics KG device notifies users or caregivers of pre-symptomatic concerns, often weeks in advance of the onset of symptoms and the presentation of illnesses such as urinary tract infection (UTI) and kidney stones.
When the risk of a condition like UTI is detected, prophylactics can be administered, a capability that may reduce the likelihood of hospitalizations.
When the risk of developing an illness including urinary tract infection (UTI) and kidney stones are recognized, prophylactics can be administered, perhaps reducing hospitalizations. For men, kidney stones can be diagnosed weeks before symptoms begin, allowing for easy treatment prior to hospitalization.
The clinical trial was meant to validate the system’s ability to detect protein in urine by testing it against over 900 samples.
TechnoSTAT, a worldwide clinical trial management and monitoring organization that conducted the research at Jerusalem’s Hadassah Ein Kerem Medical Center, discovered that KG had a sensitivity rating of 98.7% and a specificity rating of 100% for simulated five daily urinations.
According to the US National Institutes of Health (NIH), traditional urine sticks have an 80% sensitivity and a 95% specificity.
“Olive Diagnostics delivers continuous home-based, medical-grade urine diagnostics that help monitor health changes. We are enabling the early detection and treatment of certain diseases to reduce the cost of healthcare in patients and improve their quality of life. The organization is gearing up to move the device into households and clinics worldwide with a potential market of every active toilet user wishing to monitor their health,” said Guy Goldman, CEO of Olive Diagnostics.
Olive Diagnostics was founded in 2019 by CEO Guy Goldman and Corey Katz. The company has raised around $4 million in post-seed and seed money from Maccabi Healthcare Services, Cleveland Clinic, the Israel Innovation Authority, eHealth Ventures, Amgen Ventures, alongside private investors from the U.S and Europe.
The startup will raise Series A funding during the first half of 2022, in order to support its growth.
Olive Diagnostics has achieved ISO certification, a vital step toward CE certification, which will allow the company to commercialize its technology and begin sales in the European Union. Olive Diagnostics is likewise in the US FDA’s pre-submission process.




