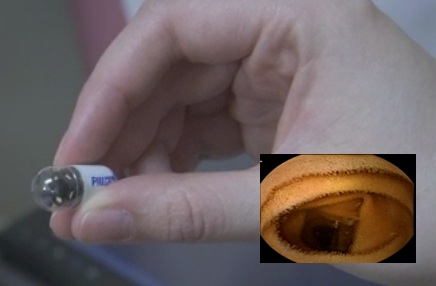
–
PillCam SB 3 will be available in the U.S. beginning in Q4 2013.
Will you offer us a hand? Every gift, regardless of size, fuels our future.
Your critical contribution enables us to maintain our independence from shareholders or wealthy owners, allowing us to keep up reporting without bias. It means we can continue to make Jewish Business News available to everyone.
You can support us for as little as $1 via PayPal at office@jewishbusinessnews.com.
Thank you.
The PillCam Capsule is about the size of a vitamin and allows for non-invasive viewing of the GI tract.
–
Given Imaging Ltd, (Nasdaq:GIVN) today announced that the United States Food and Drug Administration (FDA) has granted 510(k) clearance for the next generation PillCam, SB 3, to detect and monitor small bowel abnormalities associated with Crohn’s disease, obscure gastrointestinal (GI) bleeding and iron deficiency anemia.
“With more than 2 million procedures conducted since the first generation of the product was introduced, PillCam SB has had a significant impact on patient care in the U.S. and across the globe, ” said Homi Shamir, President and CEO, Given Imaging. “We believe PillCam SB 3 will both enhance the clinical experience for our large base of existing U.S. customers and expand the market for this product among new physicians who have not been performing PillCam procedures.”
The PillCam SB 3 system combines a 30% improvement in image resolution with adaptive frame rate technology to deliver more detailed small bowel images and coverage. In addition, PillCam SB 3’s video processing engine has been significantly improved. Proprietary algorithms in the system’s new software enable even smarter video compilation that is 40% more efficient than PillCam SB 2.
“Patients with complicated diseases involving the small bowel, such as Crohn’s disease, often struggle to comprehend what is happening inside their bodies and how it can be better managed, ” said Felice H. Schnoll-Sussman, M.D., Director, Jay Monahan Center for Gastrointestinal Health, New York-Presbyterian Weill Cornell Medical Center. “The improved image resolution and overall efficiency of the PillCam SB 3 system in capturing and analyzing images of the small bowel has potential to have a meaningful impact on patient care.”
“Our goal as we set out to reimagine and improve the PillCam platform was not just to provide physicians with more information, but to provide them with better and more actionable information. By delivering more detail, more coverage and improving the overall efficiency of our technology platform, we have achieved this with PillCam SB 3, ” said Homi Shamir. “Given Imaging is committed to helping physicians integrate PillCam SB 3 into clinical practice and improving patient access to this new innovative technology both in the U.S. and across the globe.”
The U.S. clearance of PillCam SB 3 represents the second regulatory milestone for Given Imaging in 2013. In July, the Company reported that PillCam COLON has been cleared by Japan’s Pharmaceuticals & Medical Devices Agency. PillCam SB 3 will be available in the U.S. beginning in Q4 2013.
–
About PillCam(R) SB 3
The PillCam SB 3 capsule is a minimally invasive procedure to visualize and monitor small bowel abnormalities associated with Crohn’s disease, iron deficiency anemia (IDA) and obscure GI bleeding (OGIB). The PillCam measures 11 mm x 26 mm and weighs less than four grams. Now in its third generation, PillCam SB 3 contains an imaging device and light source and transmits images at a rate between two and six images per second. Initially cleared by the U.S. Food and Drug Administration in 2001, PillCam SB is an accurate, patient-friendly tool used in patients two years and older by physicians to visualize the small bowel. PillCam SB 3 builds on Given Imaging’s unique expertise and collaborative efforts as an industry leader that includes more than 2 million uses of PillCam capsules in patients worldwide and more than 1, 900 clinical studies.
The risks of PillCam capsule endoscopy include capsule retention, aspiration and skin irritation. Endoscopic placement may present additional risks. Medical, endoscopic, or surgical intervention may be necessary to address any of these complications, should they occur.
About Given Imaging Ltd.
Since pioneering the field of capsule endoscopy in 2001, Given Imaging has become a world leader in GI medical devices, offering innovative options for visualizing, diagnosing and monitoring the digestive system.
The company offers a broad product portfolio including PillCam(R) capsule endoscope for the small bowel, esophagus and colon. The company also offers industry-leading GI functional diagnostic solutions including ManoScan(TM) high-resolution manometry, Bravo(R) capsule-based pH monitoring, Digitrapper(R) pH-Z, and the SmartPill(R) GI monitoring systems.